An FDA prior notice is a heads-up you give the FDA before shipping food products to the USA from Canada or anywhere else. It helps them make sure your products meet safety standards. Skipping this step is not an option. It can cause delays or rejections at the border, affecting your delivery schedule and profits.
Juggling compliance with the Canadian Food Inspection Agency (CFIA) and the FDA (Food and Drugs Administration) south of the border can feel like a constant game of whack-a-mole for your CPG business. Most sellers struggle with missed notices, which eventually leads to delays, frustrated customers, and potential fines. But what if there was a way to automate the entire FDA prior notice process, freeing you to focus on what matters most – growing your business?
Automated FDA notices are a game-changer for Canadian CPG companies. Imagine a system that automatically generates and sends all the necessary paperwork – prior notifications, rejection notices, detention notifications – directly to your logistics team. It has made the lives of hundreds of CPG sellers much easier.
Interested in knowing more about how to streamline compliance and keep your shipments moving smoothly, all while slashing costs? This is your chance to learn all about integrating automated FDA prior notices and how they can make CPG shipping a breeze.
Automating FDA Prior Notices for Smoother CPG Shipping
Automated FDA prior notices are the faster, more efficient equivalent of manual FDA notices. Thanks to specialized software and system integration, you don’t need to constantly scramble to keep up with your FDA notice submissions. As much as these regulations are important, most sellers often avoid them because of their time-consuming nature.
Manually scouring databases and sifting through regulations can be tedious and inefficient, especially when dealing with FDA regulations in Canada for your CPG products. To give sellers shipping their goods to the USA some relief, the FDA introduced automation.
The automated Prior Notice System Interface (PNSI) is like a super-powered assistant for FDA compliance. Automated systems leverage smart algorithms to scan FDA databases and identify notices relevant to your specific products or ingredients. Imagine receiving instant alerts the moment a new notice pops up. This will help you to put an end to the need for endless manual searches.

Manual vs. Automated FDA Notices
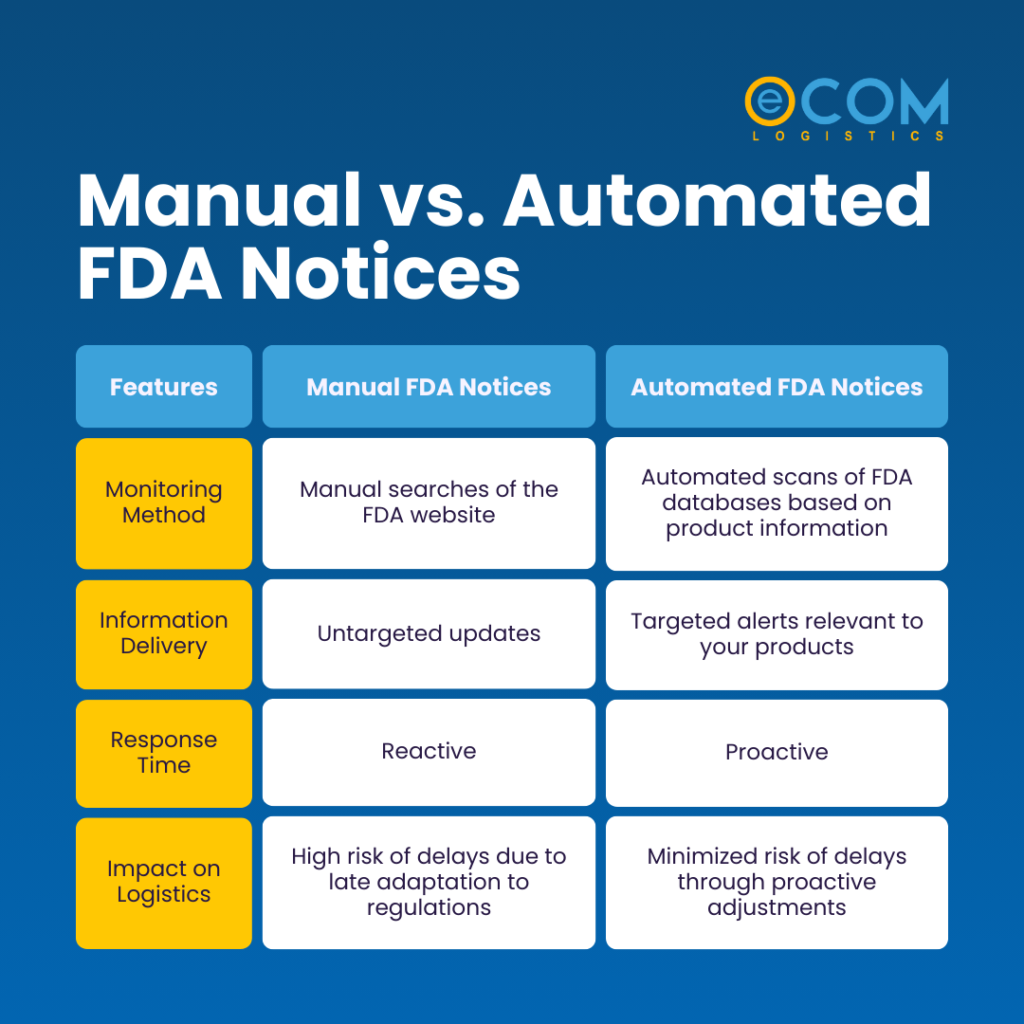
It can be difficult to understand the difference between the two types of FDA notices. But for high volume shipments, automated notices are the clear winner, ensuring streamlined FDA compliance and smoother logistics processes. Why? Let’s compare the two approaches.
Manual FDA Notices:
Manual FDA notices involve human intervention, where officials manually review documents and issue notifications. This process is slower and more labor-intensive but may be necessary for complex cases requiring expert judgment.
Imagine this:
- Endless Monitoring: You or your team dedicate precious time to scouring the FDA website for relevant notices.
- Information Overload: Going through all those FDA regulations in Canada or the USA manually and churning out the specifics related to your CPG products can be overwhelming.
- Risk of Missing Critical Updates: The manual approach means there is room for human error, which might cost you important notices. You won’t even realize when an important notice might slip through the cracks, potentially leading to non-compliance issues.
- Slow Response Times: Understanding the newly added FDA regulations takes time. It often impacts your ability to adapt logistics and potentially causes delays at the border.
Automated FDA Notices:
Automated FDA notices are electronic notifications generated by the Food and Drug Administration (FDA) in the United States. They’re essentially a better, more time-efficient version of manual notices.
Picture this:
- Effortless Monitoring: Automated systems do the heavy lifting. They scan FDA databases based on your specific product information. You’ll receive an alert the moment a relevant notice pops up.
- Targeted Information: No more information overload! You only receive alerts pertinent to your products or ingredients.
- Proactive Approach: Early awareness allows for swift action. You can assess the impact, plan adjustments (like updating labeling, etc.), and make sure that your logistics adapt accordingly. This proactive approach minimizes disruptions and keeps your shipments flowing smoothly.
Reduced Risk of Delays: You won’t need to understand all the regulatory changes by yourself. Automation will help you catch the changes early on so you can avoid potential hold-ups at the border due to non-compliance. This translates to happier customers and a healthier bottom line.
Here’s a handy table summarizing the key differences:
The bottom line is that the automated FDA prior notices are a game-changer for CPG businesses in Canada. They can help you manage your time well and minimize compliance risks. Looking at the bigger picture, the outcome of automation is keeping your products moving and your customers satisfied. It’s a win-win for everyone involved.
How to Integrate Automated FDA Prior Notices in Your CPG Logistics Operations
Now that you know how automating FDA prior notices can streamline your logistics operations, it’s time to understand the specifics of the process and learn how to adopt it in your workflow. Here’s a step-by-step guide to help you integrate automated notices successfully into your CPG logistics operations:
1. Choosing the Right Solution:
- Needs Assessment: First, identify what your specific needs are. For that, you need to consider factors such as:
- The volume of products you manage
- The complexity of your ingredients
- Your budget
- Solution Landscape: If you want to integrate the FDA prior notice system in your operations and want it to work in your favor, you need to learn about it to find compatible features, too. In order to do that, you must study various platforms to compare features like customizable alerts and reporting functionalities. You can also use software like RudiCoder to automatically submit prior notices. Thoroughly exploring your options is the best way to make sure that the features you are opting for are compatible with your existing logistics management system (LMS).
- Free Trials and Demos: The best way to find the best fit for you is the trial and error method. Don’t be afraid to leverage free trials and product demos offered by most providers. You can easily test drive the system and see if it seamlessly integrates with your workflow.
2. Powering Your Automated Compliance
- Data Accuracy: Do you remember the phrase “garbage in, garbage out?” In simple terms, the precision of your product data will determine how well your selected solution performs. Make sure that your system has access to up-to-date information on your product ingredients, formulations, and labeling. This includes details like:
- Product names
- National Drug Identification Numbers (NDINs) for drugs
- Natural Health Product Identification Numbers (NPNs) for natural health products
- Data Integration: If you want to integrate the data seamlessly, you must streamline the process! There are many solutions that offer seamless data integration with popular LMS platforms. Through this, you can easily eliminate the need for manual data entry, which will significantly reduce the risk of errors.
- Data Maintenance: Another very important step is to maintain this data. Make sure to establish a clear process for ongoing data maintenance, including regularly updating your system with any changes related to your product information. If you want your data to be absolutely accurate, you have to be diligent in this step.
3. Building a Streamlined Workflow:
- Define Roles and Responsibilities: You can have many members in your team, but you can’t rely on every one of them to do this job. But it’s not a one-person job, either. Define who within your team will be responsible for:
- Reviewing FDA prior notices
- Implementing necessary actions
- Maintaining communication with relevant stakeholders
- Communication Channels: Although you’ll need different personnel to carry out different tasks, all these jobs have to be done one after another or simultaneously at times. As such, good communication and collaboration are paramount. Make sure to establish clear communication channels for a smooth flow of work. This is important because it keeps all parties informed about new FDA notices and necessary actions.
- Standardized Procedures: Another important step is to develop a standardized operating procedure (SOP) for handling FDA prior notices. This is crucial for maintaining consistency and efficiency in your response process.
4. Training and User Adoption:
- Training Programs: Knowledge is power, and you must have skillful and knowledgeable people in your team. Equip your team with adequate knowledge and the skills required to deal with the automated FDA prior notice system. Apart from that, you must train them to:
- Interpret the information provided in the alerts
- Understand the implications for your products
- Follow established procedures
- User-Friendly Interface: The automation of FDA notices is a new development, and that’s why many are unaware of how it works. For a smooth start, make sure to choose a solution with a user-friendly interface that is easy for your team to learn and adopt. This minimizes resistance to change and also guarantees that everyone feels comfortable using the system.
5. Leveraging Your 3PL Partner:
- Expertise and Technology: A few 3PL companies in Canada offer integrated solutions that combine automated FDA notice systems with logistics management expertise. Working with a fulfillment partner can eliminate the need for in-house development and maintenance of the system, all while helping you reduce errors, enhance overall efficiency, and ensure FDA compliance.
Ecom Logistics uses advanced software to generate FDA release numbers and handle compliance processes seamlessly. In addition to this, we have our own fulfillment centers and a robust warehouse inventory tracking system, ensuring that every aspect of your logistics needs is managed with precision and reliability.
- Data Management Powerhouse: Efficient data management is crucial for maintaining compliance and operational efficiency. Ecom Logistics’ robust data management capabilities ensure your product data is accurate and seamlessly integrated with the automated FDA prior notice system through our electronic data interchange system. This integration saves you valuable time and resources by automating data entry and reducing the risk of errors.
- Compliance Support: Navigating complex FDA regulations can be challenging for businesses. Your 3PL partner’s expertise should help you manage complex regulations and minimize the risk of non-compliance issues, allowing you to focus on your core business activities. Ecom Logistics provides reliable compliance support, offering valuable guidance on interpreting FDA prior notices and implementing necessary actions to ensure hassle-free compliance with FDA regulations in Canada.
Bonus Tip: Continuous Monitoring and Improvement
Don’t set it and forget it! Regularly monitor your automated FDA prior notice system and the effectiveness of your overall compliance procedures. Be open to feedback from your team and make adjustments as needed to ensure that the system continues to meet your evolving needs.
These steps can help you easily incorporate automated FDA notifications into your CPG logistics operations. This will ultimately lead to smoother compliance, streamlined shipping, and happier customers.
For CPG product sellers, dealing with the complexities of FDA regulations in Canada and logistics can be daunting. That’s where Ecom Logistics comes in. We’re among the very few 3PL providers offering automated FDA prior notice-related solutions and are your one-stop shop for streamlined CPG logistics.

This is how we work:
- Effortless Data Integration: We use tools that seamlessly connect to your Shopify store and export order details into its Prior Notice System Interface. This process eliminates the need for manual data entry into the system, significantly saving you time and effort.
- Automated Notice Submission: Once your orders are shipped and tracking information is received, our integrated app automatically submits prior notifications to the FDA.
- Easy Labeling: The system automatically retrieves FDA release numbers for each shipment, ensuring that your products are labeled correctly and meet compliance standards.
- On-Point Workflow: We handle the complexities of FDA prior notice for you, freeing you up to focus on what matters most – running your CPG business.
Apart from this, our partnerships with the top carriers in Canada keep your products moving, whatever and wherever you’re shipping. Contact us to explore how partnering with Ecom can facilitate and streamline your CPG journey.
Frequently asked questions
1. What is an FDA Prior Notice, and why is it important in CPG logistics?
An FDA prior notice is a heads-up you give the FDA before shipping food products to the USA from Canada or anywhere else. It helps them make sure your products meet safety standards. This is a mandatory step, and skipping it can lead to shipment delays or even rejections at the border, which impacts your delivery timelines and bottom line.
2. How does automated FDA Notice integration benefit CPG logistics?
The automated FDA notice system, as the name suggests, automatically generates and submits all the necessary notices, which leads to:
- Faster clearances
- Fewer errors
- Less stress for your team
This frees your team up to focus on other priorities, like keeping inventory stocked and customers happy.
3. What challenges can arise without automated FDA Prior Notice integration?
Manual processing leaves room for human error, and it also takes a lot of time. Many sellers miss deadlines, which eventually leads to delays and loss of business as well. On top of that, inaccurate information can cause headaches with border officials. The manual process also results in your team getting bogged down in paperwork instead of focusing on strategic initiatives.
4. Is automating FDA Prior Notices expensive?
The cost of implementing an automated FDA notice system can vary depending on the features you need and the size of your business. However, the potential benefits – faster clearances, reduced errors, and increased efficiency – can significantly outweigh the investment. Many solutions offer scalable pricing plans to fit your specific needs.




